

The difference is that carbon dioxide has two oxygen atoms instead of just one.
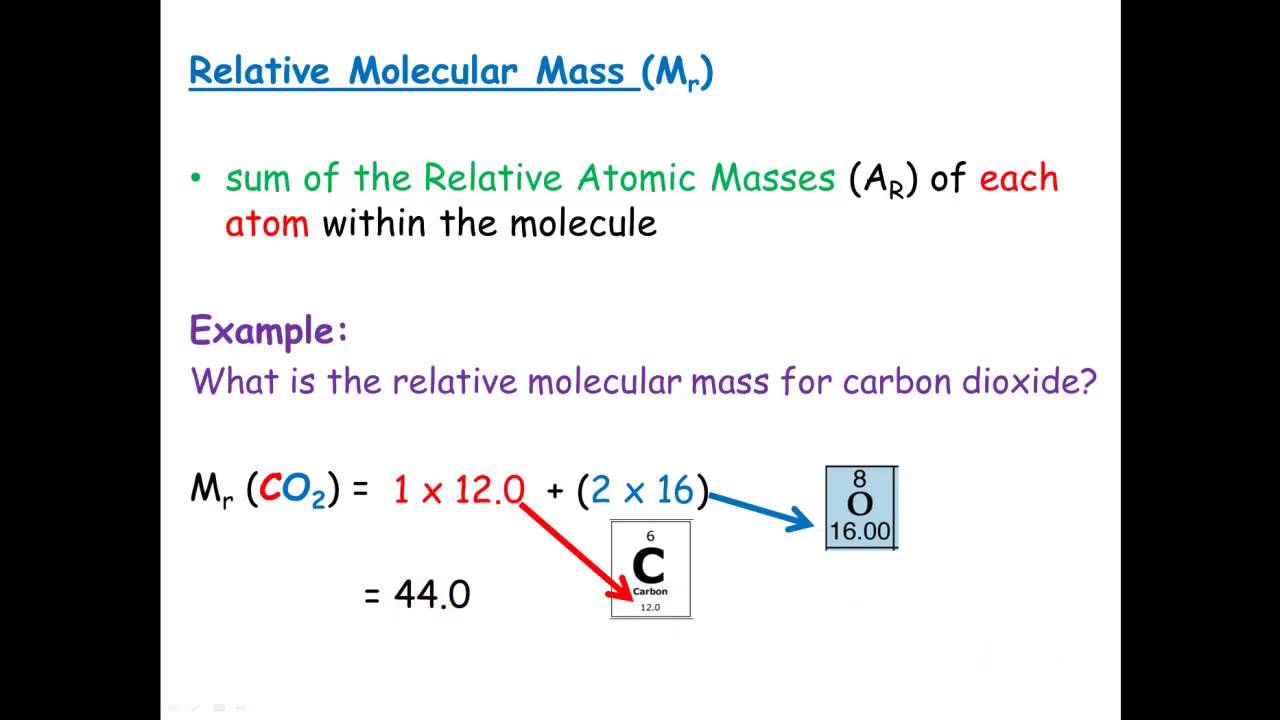
The molecular mass of CO is 12.01 + 16.00 or 28.01 g/mol.Īnother common compound with the same elements is CO 2 (carbon dioxide). You asked about CO, which is carbon monoxide, a molecular compound that has one atom of carbon (C) and one atom of oxygen (O).įrom the table, you can see that C (element number 6) has a mass of 12.01 grams per mole, and O (element number 8) has a mass of 16.00 g/mole. In that one molecule you have one atom of carbon and two atoms of oxygen. We use the term "mole" so that we don't have count all of those individual atoms or molecules (it's sort of like using "miles" to specify long distances, instead of using "inches" to describe the same distance). Think of like you have a single molecule of carbon dioxide. Sigma-Aldrich Safety Data Sheet for Carbon tetrachloride. Method: ASTM D5790 Procedure: gas chromatography/mass spectrometry Analyte: carbon tetrachloride Matrix: validated for treated drinking water. carbondioxide molecular weight Error: carbondioxide is unknown. Molar mass Molecular structure Nuclear quadrupole resonance.

A mole is a very large quantity of individual items (6.02 x 10 23). Calculate the molar mass of carbondioxide in grams per mole or search for a chemical formula or substance. To find the molecular weight of a compound (a combination of elements), you just add the weights of the elements of the elemnts of which it consists. The mass (or weight) of an element is shown in most periodic tables, beneath the symbol. To determine the molecular weight (molar mass) of carbon dioxide based on measurements of the pressure, temperature, volume and mass of a sample of the gas. The central carbon atom is joined to two oxygen atoms by covalent double bonds. It is a chart that displays important information about all of the elements that make up our universe, including a few at the end that scientists have been able to create from other elements. The chemical or molecular formula for carbon dioxide is CO 2. You should have a periodic table of the elements in your textbook, or perhaps on the wall of your classroom.


 0 kommentar(er)
0 kommentar(er)
